FAIREHR is a one-stop shop...
..for researchers to preregister their studies in the field of human and environmental health, following the FAIR (Findable, Accessible, Interoperable, and Reusable) guidance principles.
FAIREHR enhances the exchange of information on environmental and health studies, promoting validation and harmonization of monitoring approaches.
The FAIREHR aims to consolidate researches leading to generation of data on exposure to chemicals in a central IT infrastructure and to ensure that information is secure, of high quality, findable, accessible, interoperable and re-usable to the extent possible.
The FAIREHR will serve the research community from concept to project completion for generating reusable high-quality metadata and ensuring visibility throughout the research project life cycle.
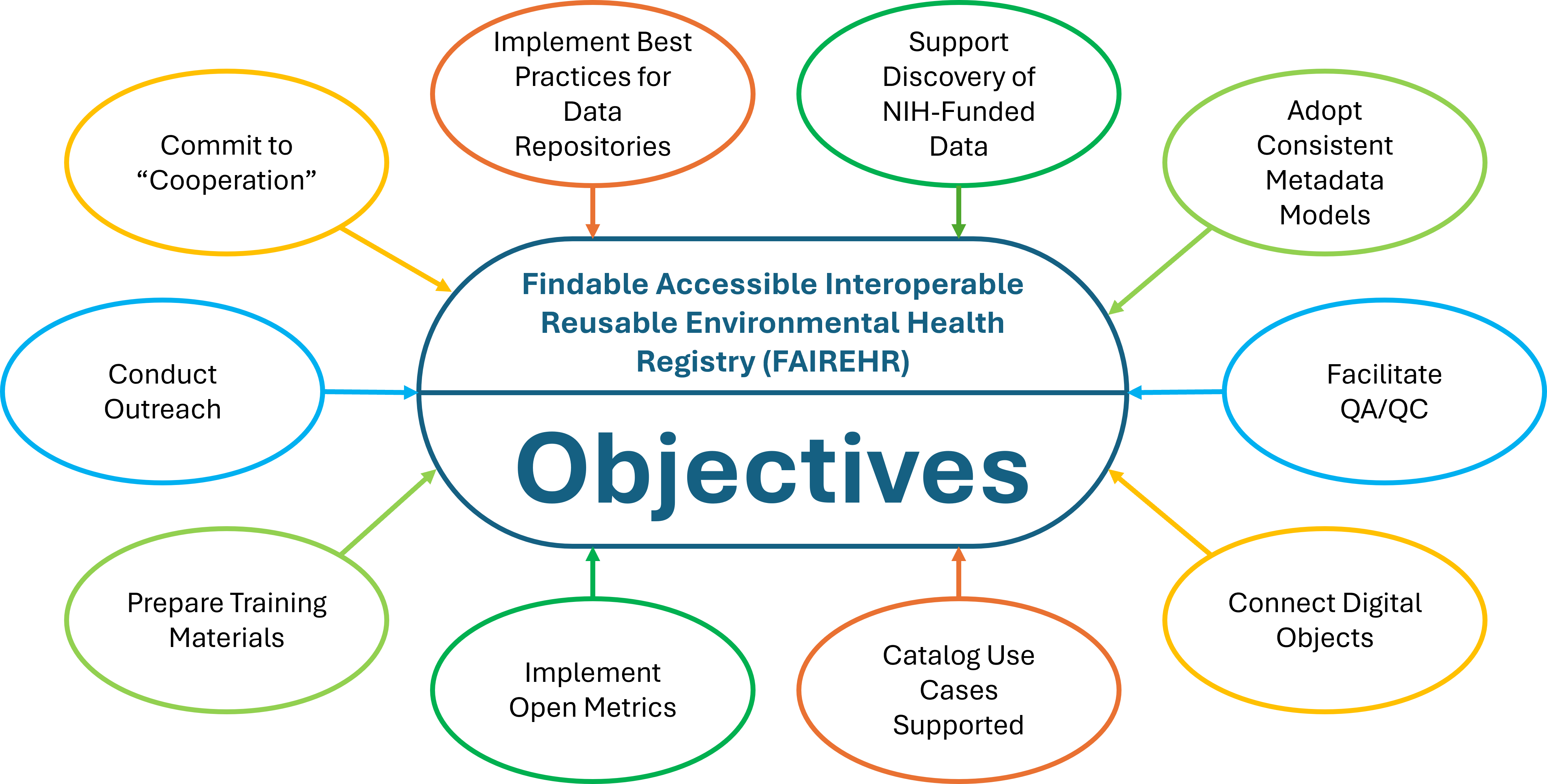
FAIREHR will:
- Comparability of research results
- Reusability of research results
- Transparency
- Replication rate
- Quality and allow study reports to be compliant with established reporting criteria
- Unnecessary duplication preventing wasted view effort and precious research funds
- Unnecessary methodological heterogeneity
- Risk of insufficient metadata collection (Metadata deficiencies)
- Selective reporting and reporting or publication bias and help shed light on negative studies
- Global collaboration between researchers
- Identification of ongoing or concluded, not yet published, studies
- Peer-reviewers to compare the manuscript findings with the registered study
- Patients and encourages patient/public participation in research
- Guideline development
- Commissioners and funders of research allowing them to identify ongoing studies in their field of interest
- Aiding the wider scientific and scholarly community by allowing for open and early peer-review of study objectives and methods, allowing for adjustments and refinement and to ensure the question asked is the right one
Browse studies
Search our database for registered studies in your field
Who should be part of the network? (users)
Preregistration of environmental and health research studies aids scientists, regulators, policy makers (at various levels such as EU, national, regional), life sciences companies, occupational health hygienist, publishing house, journals, professional associations, Contract Research Organization (CRO), Meta researchers and research funding bodies to track and identify planned and ongoing, as well as completed, studies. The metadata will also include links to related information, facilitating comprehensive data exploration.
Our platform will enable researchers to consult with experts in the field for getting insights in topics relevant for their research question. In addition, human and machine-readable metadata will enable automatic discovery of datasets and services to promote evidence-based decision-making process for research and development (R&D) and public health.

Our Mission
At FAIREHR we believe that all people must have a fair opportunity to have access to method, tools and expert knowledge to be able to generate impactful research in open science environments as fundamental enabler for public health digital transformation.
With FAIREHR we provide a unique platform for everyone to connect and be part of the smart community. FAIREHR aim is to increase or facilitate external collaborations and data integration from various sources with increasing machine discoverability and (re)use of data. Exchange information on emerging science:
Improve data quality
Promote Synergies among research teams and more efficient transfer of research findings to policy-makers and other interested stakeholders
Encourage the validation and harmonisation of common measurement methods and monitoring tools so that the demands of risk assessors can be better met
Our Vision




Objectives
Team Members
- Shahzad Rashid, IOM, Edinburgh
- Finlay Brooker, IOM, Edinburgh
- Maryam Zare Jeddi, Chair and founder
- Karen Galea
International Advisory Board
Publications and Presentations
- Zare Jeddi M, Galea KS, Viegas S, Fantke P, Louro H, Theunis J, Govarts E, Denys S, Fillol C, Rambaud L, Kolossa-Gehring M. FAIR environmental and health registry (FAIREHR)-supporting the science to policy interface and life science research, development and innovation. Frontiers in toxicology. 2023 Jun 5;5:1116707. https://www.frontiersin.org/articles/10.3389/ftox.2023.1116707/full
- Zare Jeddi M, Virgolino A, Fantke P, Hopf NB, Galea KS, Remy S, Viegas S, Mustieles V, Fernandez MF, von Goetz N, Vicente JL. A human biomonitoring (HBM) Global Registry Framework: Further advancement of HBM research following the FAIR principles. International Journal of Hygiene and Environmental Health. 2021 Sep 1;238:113826. https://doi.org/10.1016/j.ijheh.2021.113826. https://www.sciencedirect.com/science/article/pii/S1438463921001413
- Zare Jeddi M, Hopf NB, Louro H, Viegas S, Galea KS, Pasanen-Kase R, Santonen T, Mustieles V, Fernandez MF, Verhagen H, Bopp SK. Developing human biomonitoring as a 21st century toolbox within the European exposure science strategy 2020–2030. Environment international. 2022 Oct 1;168:107476. Developing human biomonitoring as a 21st century toolbox within the European exposure science strategy 2020–2030 - ScienceDirect
- Zare Jeddi M, Hopf NB, Viegas S, Price AB, Paini A, van Thriel C, Benfenati E, Ndaw S, Bessems J, Behnisch PA, Leng G. Towards a systematic use of effect biomarkers in population and occupational biomonitoring. Environment International. 2021 Jan 1;146:106257. Towards a systematic use of effect biomarkers in population and occupational biomonitoring - ScienceDirect
- de Jong E, van der Voet H, Marx‐Stoelting P, Bennekou SH, Sprong C, Bloch D, Burchardt A, Lasch A, Opialla T, Rotter S, Wedebye EB. Roadmap for action on risk assessment of combined exposure to multiple chemicals (RACEMiC). EFSA Supporting Publications. 2022 Oct;19(10):7555E. Roadmap for action on Risk Assessment of Combined Exposure to Multiple Chemicals (RACEMiC) - Jong - 2022 - EFSA Supporting Publications - Wiley Online Library
- Reale E, Jeddi MZ, Paini A, Connolly A, Duca R, Cubadda F, Benfenati E, Bessems J, Galea KS, Dirven H, Santonen T. Human biomonitoring and toxicokinetics as key building blocks for next generation risk assessment. Environment international. 2024 Feb 1;184:108474. Human biomonitoring and toxicokinetics as key building blocks for next generation risk assessment - ScienceDirect
- Viegas S, Zare Jeddi M, B. Hopf N, Bessems J, Palmen N, S. Galea K, Jones K, Kujath P, Duca RC, Verhagen H, Santonen T. Biomonitoring as an underused exposure assessment tool in occupational safety and health context—challenges and way forward. International journal of environmental research and public health. 2020 Aug;17(16):5884. Biomonitoring as an Underused Exposure Assessment Tool in Occupational Safety and Health Context—Challenges and Way Forward
- Karakoltzidis A, Battistelli CL, Bossa C, Bouman EA, Aguirre IG, Iavicoli I, Jeddi MZ, Karakitsios S, Leso V, Løfstedt M, Magagna B. The FAIR principles as a key enabler to operationalize safe and sustainable by design approaches. RSC Sustainability. 2024;2(11):3464-77. The FAIR principles as a key enabler to operationalize safe and sustainable by design approaches
- Hopf NB, Rousselle C, Poddalgoda D, Lamkarkach F, Bessems J, Schmid K, Jones K, Takaki K, Casteleyn L, Jeddi MZ, Bader M. A harmonized occupational biomonitoring approach. Environment international. 2024 Sep 1;191:108990. A harmonized occupational biomonitoring approach - ScienceDirect
- Nakayama SF, St-Amand A, Pollock T, Apel P, Ait Bamai Y, Barr DB, Bessems J, Calafat AM, Castaño A, Covaci A, Duca RC. Interpreting biomonitoring data: Introducing the international human biomonitoring (i-HBM) working group's health-based guidance value (HB2GV) dashboard. International journal of hygiene and environmental health. 2023 Jan 1;247:114046. Interpreting biomonitoring data: Introducing the international human biomonitoring (i-HBM) working group's health-based guidance value (HB2GV) dashboard - ScienceDirect
- Enhancing the use of exposure science across EU chemical policies as part of the European Exposure Science Strategy 2020–2030
- Zare Jeddi M, Boon PE, Cubadda F, Hoogenboom R, Mol H, Verhagen H, Sijm DT. A vision on the ‘foodture’role of dietary exposure sciences in the interplay between food safety and nutrition. Trends in Food Science & Technology. 2022 Feb 1;120:288-300. A vision on the ‘foodture’ role of dietary exposure sciences in the interplay between food safety and nutrition - ScienceDirect
- Exposure modelling in Europe: how to pave the road for the future as part of the European Exposure Science Strategy 2020–2030
- A human biomonitoring (HBM) Global Registry Framework: Further advancement of HBM research following the FAIR principles - ScienceDirect
- Heinemeyer G, Connolly A, von Goetz N, Bessems J, Bruinen de Bruin Y, Coggins MA, Fantke P, Galea KS, Gerding J, Hader JD, Heussen H. Towards further harmonization of a glossary for exposure science—an ISES Europe statement. Journal of Exposure Science & Environmental Epidemiology. 2022 Jul;32(4):526-9. Towards further harmonization of a glossary for exposure science—an ISES Europe statement
- The European exposure science strategy 2020–2030 - ScienceDirect
- Advancing exposure data analytics and repositories as part of the European Exposure Science Strategy 2020–2030 - ScienceDirect
- Hopf NB, Bessems J, Santonen T, Viegas S, Casteleyn L, Poddalgoda D, Lamkarkach F, Göen T, Jeddi MZ, Koller M, Rousselle C. Introducing the OECD guidance document on occupational biomonitoring: A harmonized methodology for deriving occupational biomonitoring levels (OBL). Toxicology letters. 2025 Jan 1;403:132-43. Introducing the OECD guidance document on occupational biomonitoring: A harmonized methodology for deriving occupational biomonitoring levels (OBL) - ScienceDirect
- Zare Jeddi M, Galea KS, Ashley-Martin J, Nassif J, Pollock T, Poddalgoda D, Kasiotis KM, Machera K, Koch HM, López ME, Chung MK. Guidance on minimum information requirements (MIR) from designing to reporting human biomonitoring (HBM). Environment International. 2025 Jun 16:109601. Guidance on minimum information requirements (MIR) from designing to reporting human biomonitoring (HBM) - ScienceDirect
- Framework for developing an exposure science curriculum as part of the European Exposure Science Strategy 2020–2030 - ScienceDirect
- Galea KS, Brooker F, Rashid S, Bader M, Ait Bamai Y, Bessems J, Beyene EM, Connolly A, Costa C, Deligannu P, Duca RC. FAIREHR: a novel online research registry platform to advance global environmental and occupational health research. Journal of Environmental Exposure Assessment. 2025 Sep 17;4(3):N-A. FAIREHR: a novel online research registry platform to advance global environmental and occupational